Bio-Chemistry
Carbohydrates
Carbohydrates can be used as an energy source. In plants, carbohydrates are used in the cell wall (in the form of cellulose) for structural purposes. The monomer form of carbohyrdates are monosaccharides (sugars) and the polymer form are polysaccharides (starches). monosaccharides include a single sugar (example = glucose, fructose, and galactose), diassachardies include a double sugar (example = maltose), and polysaccharides include many sugars (example = cellulose and glycogen).
The chemical composition: Carbon, Hydrogen, Oxygen. The chemical ratio of carbohyrdates is usually 1:2:1 for C:H:O; such examples can be seen in glucose (C6H12O6) or sucrose (C12H22O11).
Fun Fact: glucose and fructose have the same molecular formula (C6H12O6), but differ because they are isomers (structurally different).
Many Carbohydrates include functional groups. Here are a list of some common functional groups:
Hydroxyl: -OH
Carbonyl: C=O
Carboxyl: COOH
Amino: NH2
Sulfhydryl: -SH
Phosphate: PO4-
Methyl: CH3
Functional Groups
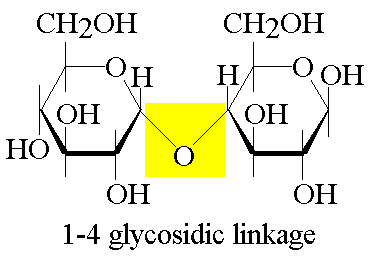
Carbohydrates are held together by glycosidic linkage. Glycosidic linkage is a covalent bond formed between two monosaccharides (carbohydrate monomer) by a process called dehydration synthesis
Glycosidic linkage: is a covalent bond formed between two monosaccharides (carbohydrate monomer) by a process called dehydration synthesis.
Dehydration Synthesis: two monomers are chemically covalently connected by the loss of an H2O molecule
In aqueous solutions, carbohydrates form ring structures.
Example: glucose
Properties of carbohydrates depends on how monomers are bonded together, usually by glycosidic linkage. Plants store starches, a polymer of glucose, used as fuel. Cellulose make up plant cell walls, and also uses glucose. But they have a slightly different ring structure. Starch (helical) = α configuration, cellulose (straight) = β configuration.
Properties and Function
Carbohydrate linkage



(The picture above represents the structure of a glucose molecule)