Bio-Chemistry
Enzyme Structure
Because enzymes are a type of protein, they are made up of amino acids (check the page on proteins). There are thousands of different kinds of organisms, even to the simplest bacteria. How can there be so many enzymes, even though there are only 20 possible amino acids? The answer is similar to how many words there are in the english language, even thought there are 26 letters in the alphabet. Different combinations of amino acids, along with the length of the polypeptide chain result in many different types of enzymes, along with different functions and structures.
What affects enzyme activity?
There are 3 enviromental factors that affect enzyme activity:
1) Temperature (usually body temperatuer, 30°C to 40°C)
2) pH (usually 6-8)
3) Salinity
-
Temperature and pH will cause denaturation of enzyme (salinity as well) With a very high temperature the thermal agitation disrupts the bonds in an enzyme and it denatures. But with a moderate increase in temperature, the enzymes work much faster (fatster rate) because heat causes the substrates to collide more frequently with active sites In cold temperatures, the molecules move slower, which decreases the number of collisions between an enzyme and substrate. Like temperature, pH will disrupt the bonds, which will also disrupt 3-D (Tertiary Structure) when an enzyme is too acidic or basic, causing denaturation to occur.
-
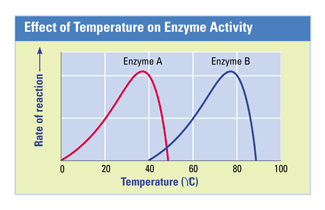
All enzymes have a 'range" at which they can operate efficently. If the enzyme goes out of that range, then it starts to denature.
The picture to the left shows such range.

Cofactors and Coenzymes
Noncompetitive and Competitve inhibition
-
Competitive inhibition is when an inhibitor mimics a substrate and blocks enzyme productivity by competing for the active site (blocking substrates from entering the active site.
-
Noncompetitive inhibition is when the inhibitors bind to a certain part of an enzyme (not the active site) which causes the enzyme to alter its shape (so that substrates can't bind; if they do bind, then the active sites will be less functional).
*Obviously, both types of inhibition will cause enzyme activity to decrease. But this isn’t necessarily a bad thing. Cells often use enzyme inhibition to regulate activity. Antibiotics are inhibitors because they block the active sites in which other organisms (like bacteria) can harmfully use.
So...what's Allosteric Regulation and Cooperativity?
Molecules can bind reversibly/irreversibly to allosteric sites. In active form, an activator will bind to where the subunits of enzymes meet. This makes a stabilized active form, which will affect all other active sites. This same concept goes for the inactive form. When activators bind to the allosteric sites, they change the activity of the enzyme.
In Cooperativity, a substance will bind to an active site of a multi sub unit enzyme, which will trigger all the active site shapes and ergo, inrease enzyme activity. A single substrate can make an enzyme act on other substrate molecules more readily. In all, the binding of one active site will affect others (which is why its considered allosteric)
* Cooperativity is an example of Activation.
* Example of Cooperativity: Hemoglobin is made up of 4 subunits with oxygen-binding sites. If an oxygen binds to one of those sites, it increases the attraction for oxygen molecules for the remaining binding sites. In areas with high levels of oxygen, more structures for oxygen is avaible as oxygen fills these binding sites.
How does Feedback Inhibition work?
Feedback inhibition is as the name describes it; an initial substrate will bind to the active site of enzymes and intermediates + end product will bind to the allosteric site. Because of this, the original enzyme will not be able to create more end products from the initial substrate (ergo, the pathway is switched off). This helps the cell from wasting chemical resources and for the cell to use good amounts of the end product.
Cofactor
Coenzyme
Nonprotein helpers for catalytic activity, which can be bound tightly to an enzyme (or not). They are inorganic
Example: Zinc, Cooper, Iron
An organic cofactor. They perform crucial chemical functions in catalysis.
Example: Vitamins