Bio-Chemistry
"Quick" Chem Review! :)
You may ask yourself, why do I need to know chemistry if I am learning biology? Well, atoms, molecules, and chemistry is the very basis of biology! To get an understanding of the big picture, one must look at the small components that make up such a model.
Atoms are composed of protons, neutrons, and electrons. All elements known to mankind are displayed in the periodic table of elements. Elements come together to form complex compounds, which is basically the beginning of the builidng blocks of life (marcromoleucles). Atoms (of different elements) have the ability to share/transfer valence electrons*, and create "bonds." It should be noted that there is no actual bond between atoms (But for the sake of simplicity, we will refer to the term "bond"); Intermolecular forces (IMFs) hold molecular compounds together, and create complex structures. Although there are many different types of IMFs, here is an example of some common forces dealt with in biochemistry:
1) Ionic Bonds: A bond that forms by the transfer of valence shell electrons between an anion and cation (oppositely charged). Ionic bonds will form ionic compounds, or salts, amongst atoms bonded.
2) Covalent Bonds: A bond that forms by the sharing of valence electrons between two cations. Covalent bonds will form a molecule amongst the atoms bonded.
3) H-bonds: A bridge that forms between polar molecules in which hydrogen atoms are connected to electronegative atoms such as Oxygen, Nitrogen, and Fluorine (𝛿+ and 𝛿-). H-bonds are typically found to be 20 times weaker than colvalent bonds.
In brief, covalent bonds share valence electrons; Ionic bonds transfer valence electrons, and H-bonds include hydrogen and oppositely charged regions attracted to one another.
*Valence electrons are unpaired electron(s) in the valence shell of an atom; such electrons determine the interactions amongst other atoms.
Intramolecular forces are forces within atoms, such as the bond between two atoms. They represent the actual bond between two atoms in a compound, and are incredibly strong. Intermolecular forces are considered to be "weak" compared to the Intramolecular forces between atoms. Examples of Intramolecular include:
1) Single bonds: A pair of valence shell electrons shared in a covalent bond-the weakest bond.
2) Double bonds: Two pairs of valence shell electrons shared in a covalent bond-they are stronger and shorter than single bonds
Click here to get a better understanding of the difference between Intramolecular forces and Intermolecular forces:
Polarity is defined as the unequal sharing of electrons. Certain atoms are defined as being electronegative. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons; Fluorine is the most electronegative atom, while Cesium remains as the least electronegative atom. The Pauling scale is used to determine the electronegativity of atoms. Polarity is usually associated with covalent bonds.
-
Nonpolar covalent bonds are bonds in which the electrons are shared and distributed equally between atoms. They can be found diatomic elements.
-
Polar covalent bonds are bonds in which the electrons are not shared equally among the atoms, due to difference in electronegativity (one pair of electrons are held more closely by a certain atom).
*The picture to the right shows the polarity and the difference of electronegativity in a water molecule.
Polarity
Isomers
Isomers are variations in the architecture of organic moleclues; they are compounds that have the same number of atoms of the same elemens, but different structures-this results in different properties! There are 3 types of isomers:
1) Structural isomers: One of several compounds that have the same structural formula but differ in the covalent arrangements of their atom (a.k.a. different structures).
2) Cis-trans Isomer: One of several compounds that have the same molecular formula and covalent bonds between atoms, but differ in the spatial arrangement of their atoms due to the inflexibility of double bonds; formerly called geometric isomer. Cis=X on the same side, Trans=X on different side.
3) Enantiomer Isomer: One of two compounds that are mirror images of each other and that differ in the shape due to the presence of an asymmetric carbon.
*The picture to the left shows an example of a Cis-trans Isomer
Moleucluar shape is critical because it allows certain molecules to bind to one another. It shows how structure relates to function within an organism. Humans can create medical advances based on how a shape of a molecule is, and how we can work off the chemical compositions as well.
Intramolecular Forces





Cis-Trans Isomer
Acids, Bases, pH, Buffers
An acid is a substance that increases the H+ concentration in a solution (loses it's H+ ion). A base reduces the H+ ion concentration. A base accepts/takes away the H+ ion in a solution, or by forming OH- ions.
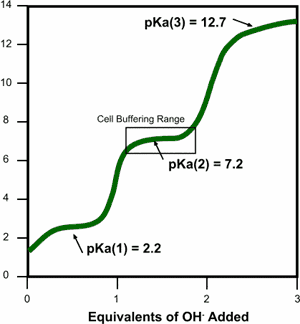
pH is defined as the negative log of a hydrogen ion concentration, used for the determination of acidity and basic of a substance. A small change in pH can have a huge impact on the H+ and OH- concentration in organisms and nature, which include altering enzymes and marcomolecules. Cells are sensitive to H+ and OH- concentrations. A small change in pH of something could increase it 100's or 1000's of times.
A buffer is a substance that allows a pH to stay at a constant rate, even with the addition of acids and bases, meaning the H+ and OH- ions. When there's a rise in pH in human blood (H+), carbonic acid will yield a bicarbonate ion and H+ concentration. Bicarbonate ion is a base. The same idea works around when there's a low pH (high OH- ion concentration) and H2CO3 is formed. Equilibrum is present for this to occur.
Acid precipitation is when rain, snow or fog precipitates on Earth, with the contents being at a much lower pH than it should (around/below 5.2). This process can damage organisms in lakes, streams, and soil chemistry.