Bio-Chemistry
Enzyme Functions
In basic terms, an enzyme's function is to lower the Activation Energy for a chemical reaction. All reactions have an activation energy (commonly abbreviated as Ea). An enzyme doesn't take part in a reaction, but merely speeds up the process, providing initial energy to overcome the energy barrier. It is often supplied in thermal form (heat) so molecules collide more forcefully and often.
Activation Energy
The example to the left shows an exergonic reaction, where energy is released to the surrounding enviornment (result). But initial energy is needed to break the bonds and the reactants go to an unstable state, called the transition state. New bonds form and energy is released to the environment. The Ea determines the rate of reaction and this reaction (to the left) occurs spontaneously.
-
The reactants AB and CD absorb enough energy from the surrondings to reach the unstable transition state, where bonds can break.

How an enzyme affects the Reaction Rate
-
One can see that at Letter A, the transition state is occuring, the energy is equivalent to Ea has been absorbed, the reactant's bond can be broken and rearranged
-
The relationship between the energy of the reactants, and the energy of the products is that the energy of the reactants is higher than the energy of the products
-
An enzyme does NOT change the ΔG symbol for the reaction; remember, an enyzme doesn't take part in the reaction!
-
Nor does an enzyme change the reactants or products of a reaction

Specifity
-
Because enzymes are specific, they can determine which chemical processes will be going on in the cell at any particular time. If all reations happened immediately, a lot of commotion will be going on, and it would be inappropriate for biological systems.
-
An enzyme's shape gives it its specifity, for the reaction it will catalyze. These proteins have unique 3-D shape/configuration, which results from the amino acid sequence.
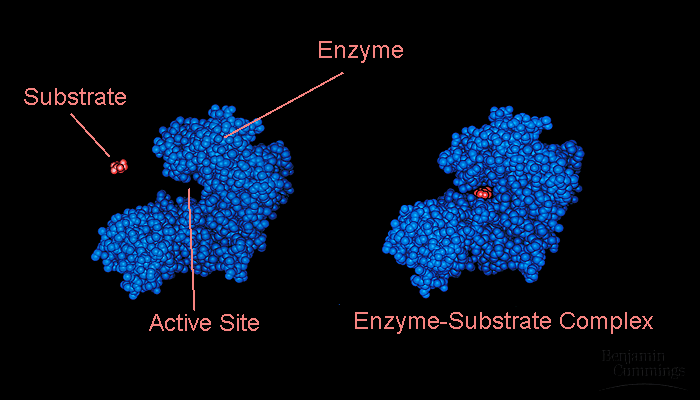
Substrates and enzymes

The Induced Fit Model: A substrate enters the active site of an enzyme. This forms weak bonds between the enzyme and substrate, due to the substrates chemcial groups + side chains, and induces a change in the shape of the enzyme. This causes the enzyme's active site to fit more "sunggly" around the substrate. The induced fit model allows chemical groups to enhance their ability.
Fun Fact
*When many substrates are available (high substrate concentration), an enzyme will become saturated when it is “filled up” with substrates; when a new product is released, a new substrate comes immediately. This is the Vmax ! The rate of the reaction is determined by how fast an enzyme can work. The only way to increase the rate of the product formation is to increase enzyme concentrations.
*Enzymes can have up to 100's, even 1,000's of active sites.
Induced Fit Model